How CRISPR Is Changing Cancer Research and
Treatment
, by NCI Staff
Ever since scientists realized that changes in DNA cause cancer, they have been searching for an easy way to correct those changes by manipulating DNA. Although several methods of gene editing have been developed over the years, none has really fit the bill for a quick, easy, and cheap technology.
But a game-changer occurred in 2013, when several researchers showed that a gene-editing tool called CRISPR could alter the DNA of human cells like a very precise and easy-to-use pair of scissors.
The new tool has taken the research world by storm, markedly shifting the line between possible and impossible. As soon as CRISPR made its way onto the shelves and freezers of labs around the world, cancer researchers jumped at the chance to use it.
“CRISPR is becoming a mainstream methodology used in many cancer biology studies because of the convenience of the technique,” said Jerry Li, M.D., Ph.D., of NCI’s Division of Cancer Biology.
Now CRISPR is moving out of lab dishes and into trials of people with cancer. In a small study, for example, researchers tested a cancer treatment involving immune cells that were CRISPR-edited to better hunt down and attack cancer.
Despite all the excitement, scientists have been proceeding cautiously, feeling out the tool’s strengths and pitfalls, setting best practices, and debating the social and ethical consequences of gene editing in humans.
How Does CRISPR Work?
Like many other advances in science and medicine, CRISPR was inspired by nature. In this case, the idea was borrowed from a simple defense mechanism found in some microbes, such as bacteria.
To protect themselves against invaders like viruses, these microbes capture snippets of the intruder’s DNA and store them away as segments called CRISPRs, or clustered regularly interspersed short palindromic repeats. If the same germ tries to attack again, those DNA segments (turned into short pieces of RNA) help an enzyme called Cas find and slice up the invader’s DNA.
After this defense system was discovered, scientists realized that it had the makings of a versatile gene-editing tool. Within a handful of years, multiple groups had successfully adapted the system to edit virtually any section of DNA, first in the cells of other microbes, and then eventually in human cells.
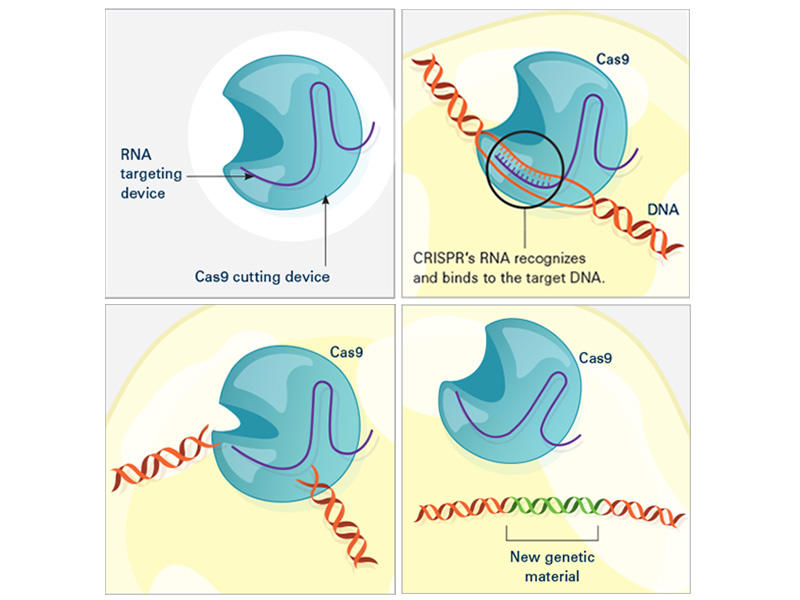
In the laboratory, the CRISPR tool consists of two main actors: a guide RNA and a DNA-cutting enzyme, most commonly one called Cas9. Scientists design the guide RNA to mirror the DNA of the gene to be edited (called the target). The guide RNA partners with Cas and—true to its name—leads Cas to the target. When the guide RNA matches up with the target gene's DNA, Cas cuts the DNA.
What happens next depends on the type of CRISPR tool that’s being used. In some cases, the target gene's DNA is scrambled while it's repaired, and the gene is inactivated. With other versions of CRISPR, scientists can manipulate genes in more precise ways such as adding a new segment of DNA or editing single DNA letters.
Scientists have also used CRISPR to detect specific targets, such as DNA from cancer-causing viruses and RNA from cancer cells. Most recently, CRISPR has been put to use as an experimental test to detect the novel coronavirus.
Why Is CRISPR a Big Deal?
Scientists consider CRISPR to be a game-changer for a number of reasons. Perhaps the biggest is that CRISPR is easy to use, especially compared with older gene-editing tools.
“Before, only a handful of labs in the world could make the proper tools [for gene editing]. Now, even a high school student can make a change in a complex genome” using CRISPR, said Alejandro Chavez, M.D., Ph.D., an assistant professor at Columbia University who has developed several novel CRISPR tools.
CRISPR is also completely customizable. It can edit virtually any segment of DNA within the 3 billion letters of the human genome, and it’s more precise than other DNA-editing tools.
And gene editing with CRISPR is a lot faster. With older methods, “it usually [took] a year or two to generate a genetically engineered mouse model, if you’re lucky,” said Dr. Li. But now with CRISPR, a scientist can create a complex mouse model within a few months, he said.
Another plus is that CRISPR can be easily scaled up. Researchers can use hundreds of guide RNAs to manipulate and evaluate hundreds or thousands of genes at a time. Cancer researchers often use this type of experiment to pick out genes that might make good drug targets.
And as an added bonus, “it’s certainly cheaper than previous methods,” Dr. Chavez noted.
What Are CRISPR’s Limitations?
With all of its advantages over other gene-editing tools, CRISPR has become a go-to for scientists studying cancer. There’s also hope that it will have a place in treating cancer, too. But CRISPR isn’t perfect, and its downsides have made many scientists cautious about its use in people.
A major pitfall is that CRISPR sometimes cuts DNA outside of the target gene—what’s known as “off-target” editing. Scientists are worried that such unintended edits could be harmful and could even turn cells cancerous, as occurred in a 2002 study of a gene therapy.
“If [CRISPR] starts breaking random parts of the genome, the cell can start stitching things together in really weird ways, and there’s some concern about that becoming cancer,” Dr. Chavez explained. But by tweaking the structures of Cas and the guide RNA, scientists have improved CRISPR’s ability to cut only the intended target, he added.
Another potential roadblock is getting CRISPR components into cells. The most common way to do this is to co-opt a virus to do the job. Instead of ferrying genes that cause disease, the virus is modified to carry genes for the guide RNA and Cas.
Slipping CRISPR into lab-grown cells is one thing; but getting it into cells in a person's body is another story. Some viruses used to carry CRISPR can infect multiple types of cells, so, for instance, they may end up editing muscle cells when the goal was to edit liver cells.
Researchers are exploring different ways to fine-tune the delivery of CRISPR to specific organs or cells in the human body. Some are testing viruses that infect only one organ, like the liver or brain. Others have created tiny structures called nanocapsules that are designed to deliver CRISPR components to specific cells.
Because CRISPR is just beginning to be tested in humans, there are also concerns about how the body—in particular, the immune system—will react to viruses carrying CRISPR or to the CRISPR components themselves.
Some wonder whether the immune system could attack Cas (a bacterial enzyme that is foreign to human bodies) and destroy CRISPR-edited cells. Twenty years ago, a patient died after his immune system launched a massive attack against the viruses carrying a gene therapy he had received. However, newer CRISPR-based approaches rely on viruses that appear to be safer than those used for older gene therapies.
Another major concern is that editing cells inside the body could accidentally make changes to sperm or egg cells that can be passed on to future generations. But for almost all ongoing human studies involving CRISPR, patients’ cells are removed and edited outside of their bodies. This “ex vivo” approach is considered safer because it is more controlled than trying to edit cells inside the body, Dr. Chavez said.
However, one ongoing study is testing CRISPR gene editing directly in the eyes of people with a genetic disease that causes blindness, called Leber congenital amaurosis.
The First Clinical Trial of CRISPR for Cancer
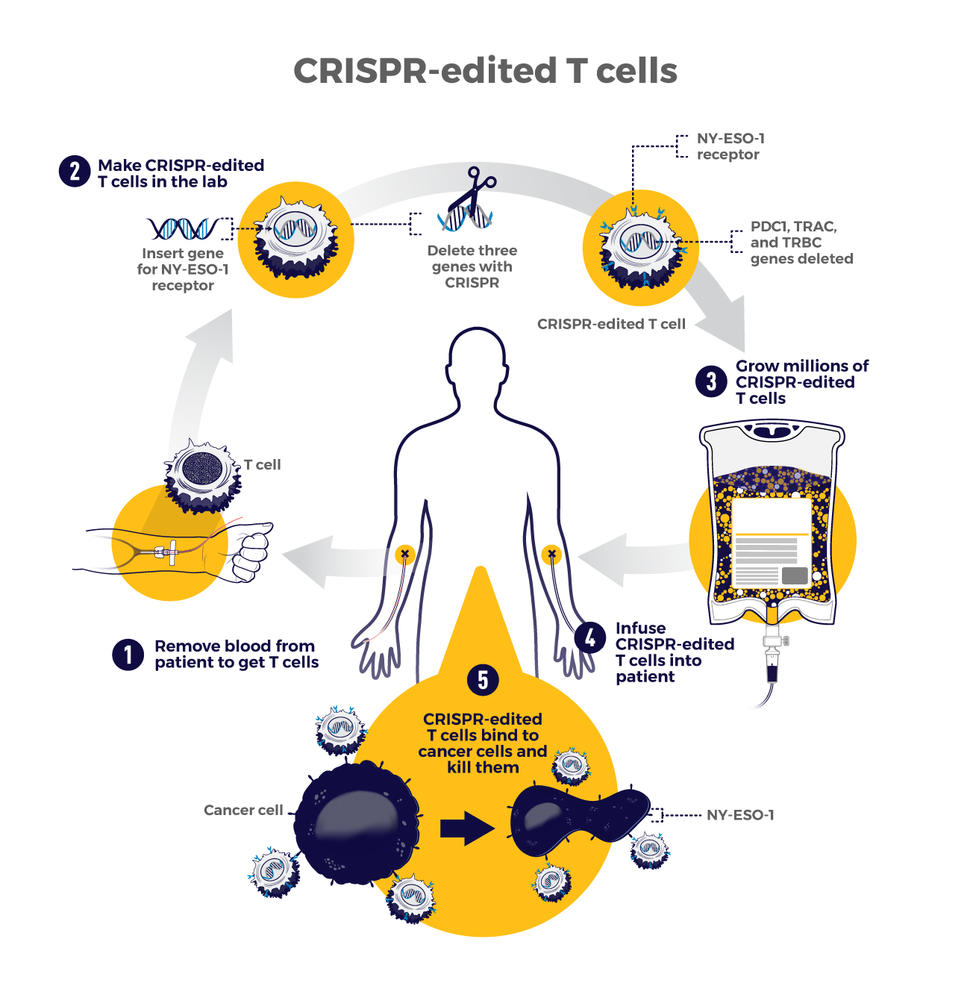
The first trial in the United States to test a CRISPR-made cancer therapy was launched in 2019 at the University of Pennsylvania. The study, funded in part by NCI, is testing a type of immunotherapy in which patients’ own immune cells are genetically modified to better “see” and kill their cancer.
The therapy involves making four genetic modifications to T cells, immune cells that can kill cancer. First, the addition of a synthetic gene gives the T cells a claw-like protein (called a receptor) that “sees” NY-ESO-1, a molecule on some cancer cells.
Then CRISPR is used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities. The finished product, dubbed NYCE T cells, were grown in large numbers and then infused into patients.
“We had done a prior study of NY-ESO-1–directed T cells and saw some evidence of improved response and low toxicity,” said the trial’s leader, Edward Stadtmauer, M.D., of the University of Pennsylvania. He and his colleagues wanted to see if removing the three genes with CRISPR would make the T cells work even better, he said.
The goal of this study was to first find out if the CRISPR-made treatment was safe. It was tested in two patients with advanced multiple myeloma and one with metastatic sarcoma. All three had tumors that contained NY-ESO-1, the target of the T-cell therapy.
Initial findings suggest that the treatment is safe. Some side effects did occur, but they were likely caused by the chemotherapy patients received before the infusion of NYCE cells, the researchers reported. There was no evidence of an immune reaction to the CRISPR-edited cells.
Only about 10% of the T cells used for the therapy had all four of the desired genetic edits. And off-target edits were found in the modified cells of all three patients. However, none of the cells with off-target edits grew in a way that suggested they had become cancer, Dr. Stadtmauer noted.
The treatment had a small effect on the patients’ cancers. The tumors of two patients (one with multiple myeloma and one with sarcoma) stopped growing for a while but resumed growing later. The treatment didn't work at all for the third patient.
It's exciting that the treatment initially worked for the sarcoma patient because “solid tumors have been a much more difficult nut to crack with cellular therapy," Dr. Stadtmauer said. "Perhaps [CRISPR] techniques will enhance our ability to treat solid tumors with cell therapies.”
Although the trial shows that CRISPR-edited cell therapy is possible, the long-term effects still need to be monitored, Dr. Stadtmauer continued. The NYCE cells are “safe for as long as we’ve been watching [the study participants]. Our plan is to keep monitoring them for years, if not decades,” he said.
More Studies of CRISPR Treatments to Come
While the study of NYCE T cells marked the first trial of a CRISPR-based cancer treatment, there are likely more to come.
“This [trial] was really a proof-of-principle, feasibility, and safety thing that now opens up the whole world of CRISPR editing and other techniques of [gene] editing to hopefully make the next generation of therapies,” Dr. Stadtmauer said.
Other clinical studies of CRISPR-made cancer treatments are already underway. A few trials are testing CRISPR-engineered CAR T-cell therapies, another type of immunotherapy. For example, one company is testing CRISPR-engineered CAR T cells in people with B cell cancers and people with multiple myeloma.
There are still a lot of questions about all the ways that CRISPR might be put to use in cancer research and treatment. But one thing is for certain: The field is moving incredibly fast and new applications of the technology are constantly popping up.
“People are still improving CRISPR methods,” Dr. Li said. “It’s quite an active area of research and development. I’m sure that CRISPR will have even broader applications in the future.”
No comments:
Post a Comment